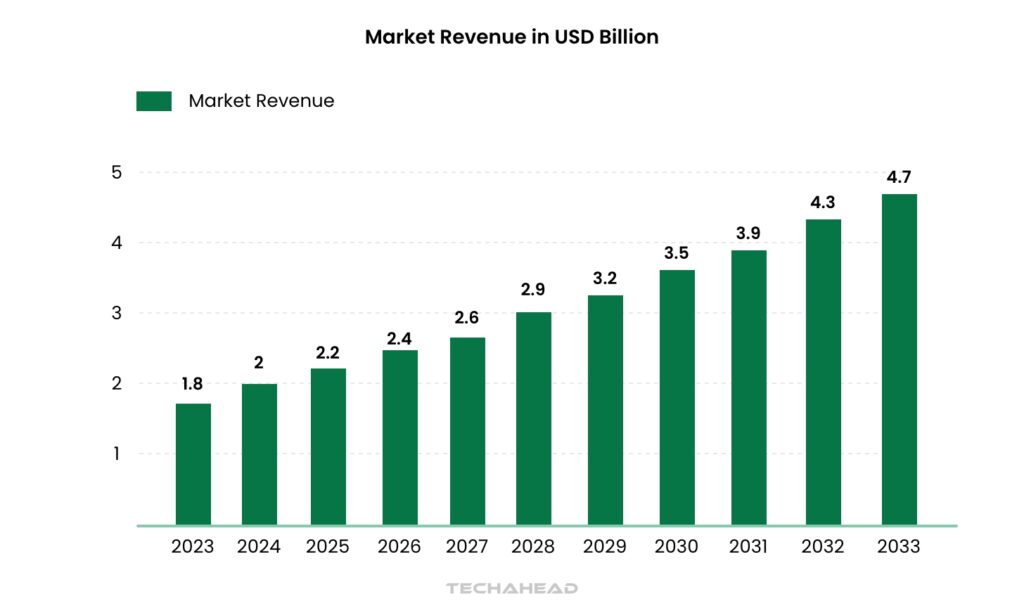
According to research, the laboratory information management system (LIMS) market is booming, with a projected value of USD 4.7 billion by 2033. This explosive growth reflects the increasing importance of LIMS for labs across various industries.
The driving force behind this trend is clear: LIMS streamlines workflows boosts data integrity, and ensures regulatory compliance.
In 2023 alone, the market size reached USD 1.8 billion, with the software and services sectors experiencing balanced growth. Pharmaceutical & biotechnology companies are leading the charge, utilizing LIMS to optimize research and development processes.
This surge in LIMS adoption presents a compelling opportunity for your lab. By embracing modernization through migration to advanced systems, strategic integrations, & custom application development, you can position yourself for success in this rapidly evolving landscape.
This blog dives deep into these key strategies, equipping you with the knowledge to unlock significant improvements in efficiency, productivity, & ultimately, scientific breakthroughs.
Why Laboratory Modernization Matters in 2025
Laboratory modernization is crucial in 2025 as the healthcare landscape rapidly evolves. With increasing demands for speed, accuracy, and accessibility, labs must adopt advanced technologies like Laboratory Information Management Systems (LIMS) and automation to streamline operations and reduce errors.
Modernization enhances patient experiences through digital interactions, such as online bookings and automated notifications, fostering trust and satisfaction.
Furthermore, scalable technology supports growth, enabling labs to manage increased workloads efficiently. In a competitive market, leveraging cutting-edge solutions positions labs as industry leaders, ensuring they not only survive but thrive in the future of diagnostics.
The True Cost of Outdated Lab Systems
Imagine scientists spending hours searching for reagents or manually logging data instead of focusing on groundbreaking research. This is the reality for many labs clinging to outdated inventory management systems.
While manual methods might seem familiar, they come at a hidden cost that eats away at productivity, budgets, and even data integrity.
This inefficiency starts with wasted time. Scientists grapple with disorganized inventory, leading to lost reagents and time spent searching. Manual data entry further slows them down, diverting them from research tasks.
These delays can have a domino effect, extending research timelines and impacting project completion.
But time isn’t the only casualty. Outdated systems can lead to overstocking, resulting in wasted resources when reagents expire before use. Inaccurate records also raise compliance concerns, risking data integrity and project validity.
Additionally, the stress of manual tasks can contribute to lower employee morale and even turnover.
The solution lies in embracing user-friendly digital inventory systems. The following section will detail the key benefits that digital transformation brings to laboratory environments.
Key Benefits of Digital Transformation in Labs
Digital transformation in laboratory environments represents a paradigm shift from traditional manual processes to integrated, intelligent systems. This evolution, driven by Lab 4.0 principles, delivers unprecedented advantages in efficiency, accuracy, and innovation potential.
By implementing digital solutions, laboratories can dramatically reduce human error while simultaneously increasing sample throughput and data quality.
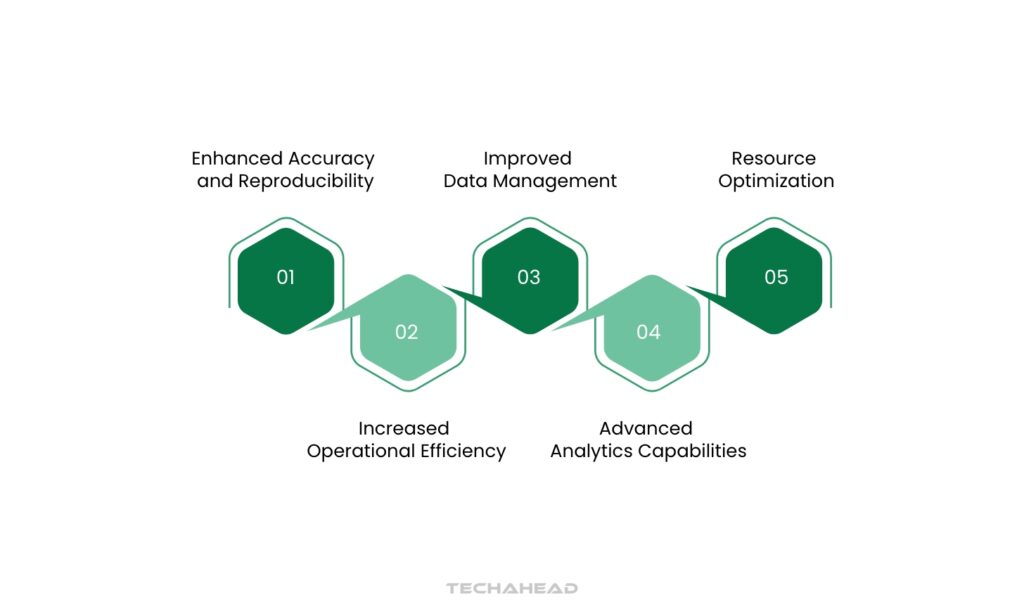
The integration of robotics, artificial intelligence, and cloud computing creates a synergistic ecosystem where routine tasks are automated, data is analyzed in real-time, and insights are generated faster than ever before. Key benefits include:
Enhanced Accuracy and Reproducibility
Automated systems eliminate human variability in procedures like titration and pipetting, ensuring consistent results across all analyses
Increased Operational Efficiency
Robotics and AI-driven automation handle routine tasks, allowing staff to focus on complex analysis and strategic initiatives
Improved Data Management
Cloud solutions enable seamless data storage, sharing, and access from anywhere in the world, facilitating global collaboration
Advanced Analytics Capabilities
Big Data integration reveals patterns and correlations that would be impossible to detect through traditional methods
Resource Optimization
Lean Lab concepts reduce waste and streamline processes, leading to significant cost savings and improved resource allocation
Up next, we’ll explore the Compliance and Regulatory Advantages of Modern Lab Systems, where we’ll discover how digital transformation helps laboratories maintain stringent quality standards while meeting evolving regulatory requirements.
Compliance & Regulatory Advantages of Modern Lab Systems
Maintaining regulatory compliance is a critical aspect of laboratory operations, especially in industries governed by strict standards like pharmaceuticals and biotechnology. Modern lab systems offer significant advantages in this area, streamlining compliance efforts and minimizing the risk of costly violations.
These systems help laboratories adhere to various industry standards, including ISO, GLP (Good Laboratory Practices), and FDA regulations. A key feature is the automated tracking of audit trails.
These digital records provide a comprehensive history of every action performed within the system, including who made changes, when they were made, and what was changed. This detailed tracking is invaluable during audits, allowing for quick and easy retrieval of information and demonstrating adherence to regulatory requirements.
Furthermore, modern lab systems provide secure and centralized storage for all required documentation. This ensures that all necessary records are readily available and properly maintained, making the laboratory consistently audit-ready.
By automating these essential compliance tasks, modern lab systems not only save valuable time and resources but also significantly reduce the risk of human error and ensure data integrity, which is paramount for regulatory compliance.
Now that we’ve explored the compliance and regulatory advantages, in the next section, Essential Steps for Lab Digital Transformation, we’ll discuss how to effectively implement these modern systems.
Essential Steps for Lab Digital Transformation
To successfully navigate the digital transformation of your laboratory, consider these essential steps:
Assessing Your Current Lab Infrastructure
Begin by evaluating your existing systems, processes, and technology. This includes conducting a gap analysis to identify inefficiencies and areas for improvement.
Understanding your data management practices and IT architecture is crucial to pinpoint where digital solutions can have the most impact.
Creating a Lab Modernization Roadmap
Develop a strategic plan that outlines your digital transformation goals, timelines, and priority areas. This roadmap should detail specific objectives, such as improving turnaround times or enhancing data accuracy.
By breaking down the transformation into manageable phases, you can ensure a structured approach that aligns with your lab’s needs.
Building Your Lab Modernization Budget
Establish a realistic budget that accounts for technology investments, training, and potential operational disruptions during the transition. Consider both initial costs and long-term benefits to justify your investment.
Engaging stakeholders in this process will help secure necessary resources and foster a culture of collaboration.
By following these steps, laboratories can effectively embrace digital transformation, enhancing efficiency and positioning themselves for future growth in an increasingly competitive landscape.
Laboratory Information Management System (LIMS) Migration
Migrating to a new Laboratory Information Management System (LIMS) is a complex undertaking, but it’s often necessary to improve efficiency, scalability, and compliance. A well-planned migration strategy is crucial for a smooth transition and minimal disruption to laboratory operations. This section will cover key aspects of LIMS migration, including planning, common challenges, and best practices.
Planning Your LIMS Migration Strategy
A Laboratory Information Management System (LIMS) migration represents a critical transformation in any lab’s operations. Success hinges on methodical planning that addresses both technical requirements and organizational dynamics.
The planning phase serves as the cornerstone of the entire migration process, determining not just the immediate transition but the long-term effectiveness of the new system. The essential components of LIMS migration planning encompass:
Define Objectives and Scope
- Establish clear, measurable goals for the migration project
- Document specific pain points in the current system that need addressing
- Create a prioritized list of must-have versus nice-to-have functionalities
- Set realistic boundaries to prevent scope creep and maintain project focus
- Align migration objectives with broader organizational strategic goals
Current System and Data Assessment
- Conduct a comprehensive audit of existing LIMS capabilities
- Map current data structures, workflows, and integration points
- Evaluate data quality and identify potential migration challenges
- Document custom configurations and specialized processes
- Create an inventory of reports and queries that need to be transferred
- Assess the impact on existing integrations with other laboratory systems
LIMS Vendor and System Selection
- Develop detailed vendor evaluation criteria
- Assess potential systems for technical compatibility
- Evaluate vendor track record and support capabilities
- Consider the total cost of ownership, including maintenance and updates
- Review vendor stability and long-term viability
- Examine system scalability and future expansion capabilities
Migration Plan Development
- Create a detailed project timeline with key milestones
- Define specific data migration strategies and methodologies
- Establish testing protocols and acceptance criteria
- Plan user training programs and documentation requirements
- Develop contingency plans for potential challenges
- Create rollback procedures in case of critical issues
Project Team and Governance
- Assign roles and responsibilities to team members
- Create clear communication channels and reporting structures
- Establish decision-making protocols and escalation paths
- Define success metrics and monitoring procedures
- Set up regular review meetings and progress tracking
- Ensure stakeholder representation across all affected departments
The success of a LIMS migration fundamentally depends on the quality and thoroughness of this planning phase. Each element must be carefully considered and documented, with contingencies built in for potential challenges.
This comprehensive approach ensures that when execution begins, the team has a clear roadmap to follow and the necessary tools to address any obstacles that arise.
Common LIMS Migration Challenges and Solutions
Migrating to a new Laboratory Information Management System (LIMS) can be a complex process, often presenting several challenges that labs must navigate effectively. Here are the common hurdles faced during LIMS migrations and strategies to overcome them:
Data Migration Complexity:
The transition involves moving large volumes of data, which can be intricate and time-consuming. To mitigate this, conduct thorough data mapping, cleansing, and transformation before the migration. This preparation ensures that data is accurately transferred to the new system.
Data Validation:
Ensuring the accuracy and integrity of migrated data is critical. Implement rigorous validation procedures to identify and rectify any discrepancies. This step helps maintain data quality and reliability in the new LIMS.
User Adoption and Training:
Resistance from users can impede the successful implementation of a new system. To foster acceptance, provide comprehensive training and ongoing support. Engaging users early in the process can also help ease their transition to new workflows.
System Integration:
Integrating the new LIMS with existing lab systems, such as Electronic Lab Notebooks (ELNs) and instruments, can pose technical challenges. Planning for integration early in the migration process and collaborating closely with vendors ensures compatibility and smooth operation.
Downtime and Business Disruption:
Migrations may lead to operational downtime, affecting lab productivity. To minimize disruption, schedule migrations during off-peak hours and develop contingency plans to address potential issues.
By proactively addressing these challenges through careful planning and execution, laboratories can achieve a successful LIMS migration that enhances efficiency and supports future growth.
Best Practices for Data Migration and Validation
Data migration and validation are paramount to a successful LIMS migration. Ensuring data accuracy and consistency in the new system is crucial for maintaining data integrity and avoiding downstream issues. Here are key best practices to follow:
Data Mapping
Begin by creating a comprehensive map of all data fields from your legacy system to the new LIMS. This detailed mapping outlines how each data element will be transferred and where it will reside in the new database. This step is essential for ensuring data is correctly placed and accessible after migration.
Data Cleansing and Transformation
Before migrating data, it’s crucial to cleanse and transform it. This involves identifying and correcting any errors, inconsistencies, or redundancies in the existing data. Data transformation may include converting data formats, standardizing units of measurement, and ensuring data conforms to the new LIMS’s data structure. This step ensures data consistency and accuracy in the new system.
Data Validation Testing
Rigorous testing is essential to validate the migrated data. This includes several types of testing:
- Unit Testing: Testing individual data elements to ensure they have been migrated correctly.
- Integration Testing: Testing the interaction of migrated data with other parts of the new LIMS.
- User Acceptance Testing (UAT): Allowing end-users to test the migrated data in real-world scenarios to ensure it meets their needs and workflows.
- Phased Migration: A phased migration approach can minimize risk and disruption to laboratory operations. This involves migrating data and functionalities in stages, allowing for thorough testing and validation at each step. This approach allows for quicker identification and resolution of any issues that arise during the migration.
Documentation
Comprehensive documentation of the entire migration process is crucial. This includes detailed records of data mapping, transformation rules, validation procedures, and testing results. This documentation serves as a valuable resource for troubleshooting, auditing, and future healthcare system maintenance.
By meticulously planning and executing these data migration and validation steps, laboratories can minimize disruptions to operations, ensure the integrity of their data, and maximize the return on their LIMS investment.
A well-executed data migration sets the stage for a successful transition to the new system and unlocks its full potential for improved laboratory efficiency and data management.
Critical Lab Systems Integration
Modern laboratories rely on a complex network of interconnected systems to manage data, automate processes, and drive scientific discovery. Integrating these systems effectively is crucial for maximizing efficiency, minimizing errors, and ensuring data integrity.
This section explores key strategies for integrating critical lab systems, including ELNs, LIMS, instruments, analytics platforms, and cloud technologies.
ELN and LIMS Integration
Electronic Lab Notebooks (ELNs) and Laboratory Information Management Systems (LIMS) are two essential tools in modern labs. ELNs capture experimental data, procedures, and observations, while LIMS manage samples, workflows, and analytical results. Integrating these systems offers several key benefits:
- Seamless Data Flow: Integration eliminates manual data entry between ELNs and LIMS, reducing errors and saving time. Data captured in the ELN can be automatically transferred to the LIMS, ensuring data consistency and traceability.
- Improved Workflow Efficiency: Integrated workflows streamline lab processes, such as sample tracking, testing, and reporting. This automation reduces manual steps and improves overall efficiency.
- Enhanced Data Integrity: Integration ensures data consistency and reduces the risk of data duplication or errors. This enhances data integrity and supports regulatory compliance.
- Better Collaboration: Integrated systems facilitate better collaboration among researchers by providing a centralized platform for accessing and sharing data.
Strategies for ELN and LIMS Integration:
- API-Based Integration: Using Application Programming Interfaces (APIs) allows for seamless data exchange between ELNs and LIMS. This approach offers flexibility and customization.
- Middleware Solutions: Middleware acts as a bridge between different systems, facilitating data exchange and integration. This can be a good option for integrating systems with limited API capabilities.
- Standard Data Formats: Using standard data formats, such as XML or JSON, ensures compatibility between systems and simplifies data exchange.
Connecting Instruments and Analytics Platforms
Integrating lab instruments and analytics platforms with LIMS and ELNs is essential for automating data acquisition and analysis. This integration offers several advantages:
- Automated Data Capture: Direct integration eliminates manual data entry from instruments, reducing errors and improving data accuracy.
- Real-Time Data Analysis: Integrating analytics platforms allows for real-time data analysis and visualization, providing immediate insights into experiments.
- Improved Data Traceability: Linking instrument data to samples and experiments in the LIMS and ELN ensures complete data traceability and supports audit trails.
Strategies for Instrument and Analytics Platform Integration:
- Instrument Interfaces: Many modern instruments offer interfaces for direct connection to LIMS and ELNs.
- Data Acquisition Systems: Data acquisition systems can collect data from multiple instruments and transfer it to the LIMS or ELN.
- Software Development Kits (SDKs): SDKs provide tools and libraries for developing custom integrations between instruments and other systems.
Cloud Integration for Modern Labs
Cloud computing offers numerous advantages for lab systems integration, including scalability, accessibility, and cost-effectiveness. Key benefits of cloud integration include:
- Centralized Data Storage and Access: Cloud-based systems provide centralized data storage and access, facilitating collaboration and data sharing among researchers across different locations.
- Scalability and Flexibility: Cloud platforms can easily scale to accommodate growing data volumes and changing business needs.
- Cost Savings: Cloud computing eliminates the need for significant upfront investments in hardware and infrastructure.
- Improved Security and Compliance: Cloud providers invest heavily in security measures and comply with industry regulations, ensuring data protection and compliance.
Strategies for Cloud Integration:
- Cloud-Based LIMS and ELN: Implementing cloud-based LIMS and ELN solutions simplifies integration with other cloud-based systems and provides easy access to data from anywhere.
- Cloud APIs: Utilizing cloud APIs allows for seamless integration between different cloud services and on-premise systems.
- Hybrid Cloud Approach: A hybrid cloud approach combines on-premise and cloud resources, offering flexibility and control over data storage and processing.
By implementing effective integration strategies, laboratories can create a seamless and efficient data ecosystem that supports scientific discovery and drives innovation. This integration not only streamlines workflows and reduces errors but also enhances data integrity, facilitates collaboration, and improves overall lab productivity.
Custom Laboratory Application Development
Custom laboratory application development creates tailored software solutions to address specific lab needs. These applications can automate workflows, manage data, integrate instruments, and enhance collaboration, ultimately improving efficiency, accuracy, and compliance within the lab environment.
When to Choose Custom Lab Solutions
Choosing custom lab solutions is essential when standard systems fail to meet the unique needs of your laboratory. If your lab has specialized workflows, specific regulatory requirements, or unique data management needs that off-the-shelf solutions cannot adequately address, custom solutions become a necessity.
Additionally, if you anticipate significant growth or changes in your operations, a tailored system can provide the scalability and flexibility required to adapt over time. Custom solutions also allow for seamless integration with existing technologies, ensuring a cohesive workflow.
Ultimately, investing in a bespoke system can enhance efficiency, improve data accuracy, and support the specific objectives of your lab, making it a strategic choice for long-term success.
Key Features of Modern Lab Applications
Modern laboratory applications represent the cornerstone of digital transformation in research and testing environments. These sophisticated platforms combine automation, data management, and analytical capabilities to streamline laboratory operations and enhance research outcomes.
Data Management and Analytics
- Real-time data capture and processing
- Advanced analytics dashboards with customizable visualizations
- Automated data validation and quality control checks
- Secure data storage with role-based access controls
- Integration with data lakes and warehouse systems
Workflow Automation
- Customizable workflow templates for different experiment types
- Automated sample tracking and management
- Instrument integration and control capabilities
- Automated scheduling and resource allocation
- Built-in quality control checkpoints
Collaboration Tools
- Real-time collaboration features for team members
- Secure document sharing and version control
- Electronic lab notebook integration
- Remote access capabilities
- Audit trail tracking for all user activities
Compliance and Security
- Built-in regulatory compliance features (GMP, FDA, ISO)
- Electronic signatures and approval workflows
- Comprehensive audit trails
- Data encryption and security protocols
- Automated compliance reporting
Integration Capabilities
- API-first architecture for seamless system integration
- Instrument connectivity and data transfer
- ERP and LIMS integration capabilities
- Cloud compatibility for scalable operations
- Mobile device support and accessibility
User Experience
- Intuitive, modern user interface
- Configurable dashboards and reports
- Role-based access control
- Mobile-responsive design
- Minimal training requirements
These features collectively ensure that modern lab applications not only meet current operational needs but also provide the flexibility and scalability required for future growth and adaptation to emerging technologies and regulatory requirements.
Selecting the Right Development Partner
Selecting the right Laboratory Custom App Development company is crucial for the success of your lab’s custom solutions. Start by evaluating potential partners based on their expertise in laboratory systems and familiarity with your specific industry requirements.
Look for a partner with a proven track record of delivering similar projects and strong references from past clients. Effective communication is essential; ensure they understand your goals and can provide ongoing support throughout the development process.
Additionally, assess their technical capabilities, including proficiency in relevant technologies and integration experience with existing lab systems.
Finally, consider their approach to project management and collaboration, as a strong partnership will facilitate a smoother development process and ultimately lead to a solution that meets your lab’s unique needs.
Future-Proofing Your Laboratory
Future-proofing a laboratory requires a strategic approach that balances technological advancement with practical implementation. A forward-thinking strategy ensures laboratories remain competitive while maximizing return on investment in new technologies and processes.
Emerging Technologies in Lab Automation
The automation landscape is rapidly evolving with cutting-edge technologies:
- AI-powered laboratory systems for predictive maintenance and workflow optimization
- Advanced robotics for high-precision sample handling and analysis
- Internet of Things (IoT) sensors for real-time monitoring and data collection
- Machine learning algorithms for pattern recognition and result prediction
- Digital twins for process simulation and optimization
- Augmented reality systems for guided procedures and training
- Cloud-based laboratory orchestration platforms
Scalability and Growth Planning
Strategic planning for laboratory expansion requires:
- Modular laboratory design that accommodates future equipment additions
- Flexible infrastructure that supports increased workflow capacity
- Scalable data storage solutions with cloud integration
- Expandable automation systems that grow with demand
- Adaptable workspace configurations for changing needs
- Resource allocation models that anticipate growth
- Technology stack that supports horizontal and vertical scaling
Training and Change Management Strategies
Successful implementation of new technologies depends on effective personnel development:
- Comprehensive training programs for all skill levels
- Continuous education initiatives to keep pace with technological advances
- Cross-training opportunities to ensure operational resilience
- Digital learning platforms for self-paced skill development
- Mentorship programs to transfer knowledge effectively
- Regular assessment of training effectiveness
- Change management frameworks to ensure smooth transitions
Each of these areas requires careful consideration and planning and implementation considerations:
- Regular technology audits to identify upgrade opportunities
- Clear ROI metrics for new technology investments
- Phased implementation approaches to minimize disruption
- Feedback loops for continuous improvement
- Risk assessment and mitigation strategies
- Vendor evaluation and partnership development
- Compliance monitoring and adaptation
Success Factors:
- Strong leadership commitment to innovation
- Clear communication channels across all levels
- Adequate resource allocation for training and development
- Regular review and adjustment of strategies
- Employee engagement in change initiatives
- Data-driven decision-making processes
- Proactive approach to regulatory compliance
By addressing these key areas comprehensively, laboratories can build resilient operations that adapt to changing requirements while maintaining efficiency and quality standards. Regular review and updates of these strategies ensure continued alignment with organizational goals and industry developments.
Implementation Success Stories
One notable case is the collaboration with DaVita Labs, which focused on consolidating and automating their diagnostic laboratory operations.
By integrating Siemens’ Atellica Solution, DaVita Labs enhanced its testing capacity, achieving a 36% improvement in system uptime. This was accomplished through predictive maintenance analytics, allowing for timely interventions and reduced downtime.
ROI and Performance Metrics
The implementation of the Atellica Process Manager at Singing River Health System resulted in a dramatic increase in workflow efficiency and overall quality. The system enabled the lab to manage samples more effectively, leading to faster turnaround times and improved patient outcomes. Performance metrics indicated a marked reduction in errors, with turnaround times decreasing by as much as 77%.
Lessons Learned from Successful Migrations
Key lessons from these implementations include the importance of change management and staff training. Successful migrations emphasized proactive service models over reactive ones, ensuring continuous support and maintenance throughout the transition phase.
Siemens’ collaboration with analytics partners like SAS has been instrumental in developing robust predictive maintenance frameworks that adapt to evolving laboratory needs.
These case studies underscore Siemens Healthineers’ commitment to enhancing laboratory operations through innovative automation solutions, ultimately leading to improved healthcare delivery.
Conclusion
Modernizing your lab through LIMS migration, strategic integrations, and custom app development is a transformative investment. These upgrades streamline workflows, enhance data integrity, and ensure regulatory compliance. By carefully planning each stage, from migration strategy to integration implementation, and considering custom app development for specific needs, you can unlock significant improvements in efficiency, productivity, and ultimately, scientific discovery. Embracing these modern solutions positions your lab for continued success in today’s rapidly evolving scientific landscape.

FAQs
The timeline for modernizing your laboratory can vary based on how extensive and complex the project is. A simple system migration might take around 3 to 6 months, while a more comprehensive upgrade that includes custom applications and multiple integrations usually takes about 8 to 12 months. Several factors can influence this timeline, such as your current infrastructure, the amount of data you have, any regulatory requirements you need to meet, and the training needs of your staff.
Modernization costs usually cover software licenses, hardware upgrades, integration and custom app development, staff training, compliance documentation, and ongoing maintenance and support to ensure smooth operations.
When selecting custom applications for our laboratory, we should consider specific workflow needs, integration capabilities, scalability, user experience, regulatory compliance, total cost of ownership, and the vendor’s expertise and support.